
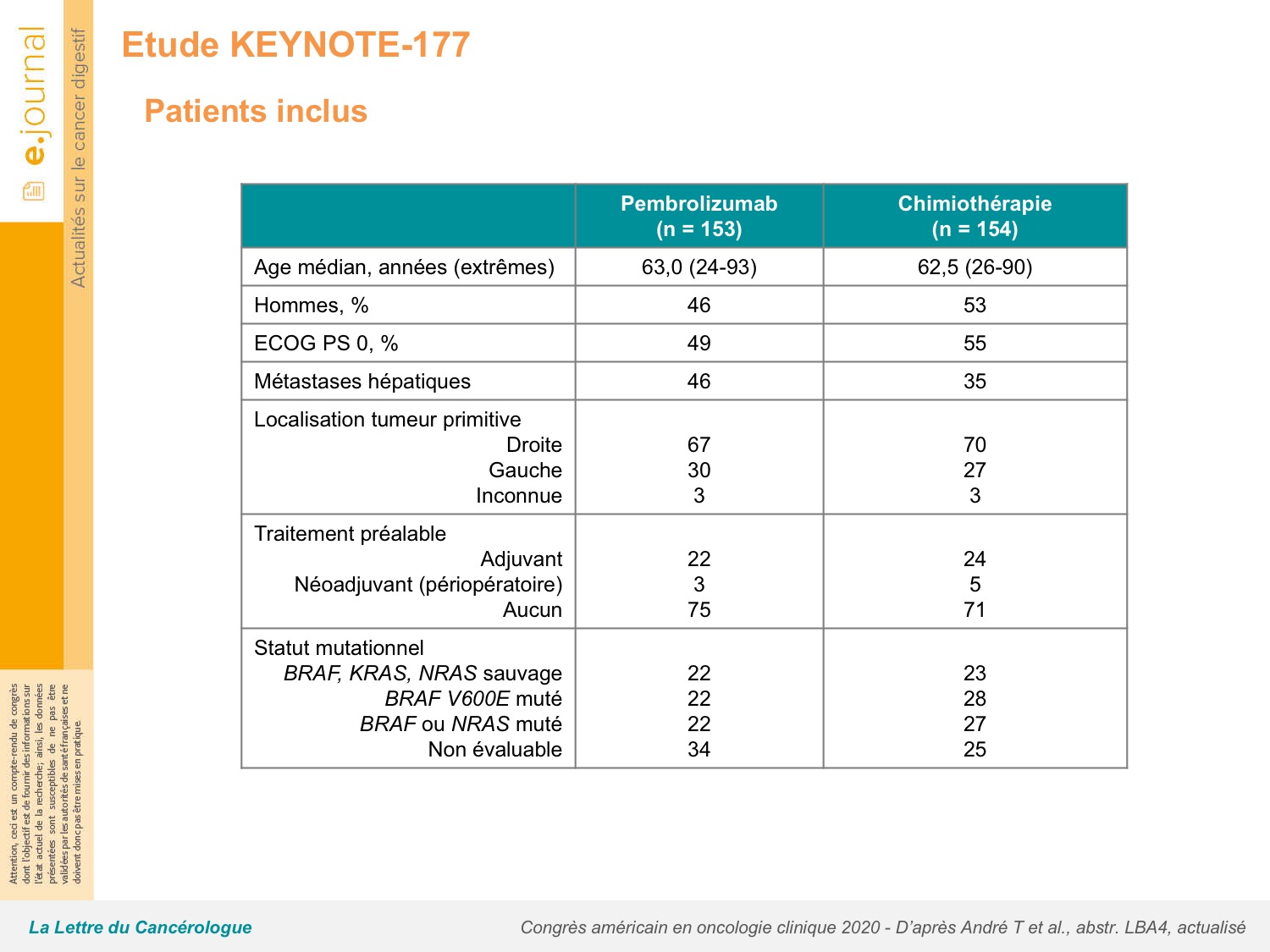
Common adverse events of grade 3 or worse that were attributed to pembrolizumab were increased alanine aminotransferase, colitis, diarrhoea, and fatigue in three (2%) patients each, and those attributed to chemotherapy were decreased neutrophil count (in 24 patients), neutropenia (22 ), diarrhoea (14 ), and fatigue (13 ). Treatment-related adverse events of grade 3 or worse occurred in 33 (22%) of 153 patients in the pembrolizumab group versus 95 (66%) of 143 patients in the chemotherapy group. At this updated analysis, median progression-free survival was 16♵ months (95% CI 5♴-38♱) with pembrolizumab versus 8♲ months (6♱-10♲) with chemotherapy (HR 0♵9, 95% CI 0♴5-0♷9). Superiority of pembrolizumab versus chemotherapy for overall survival was not demonstrated because the prespecified α of 0♰25 needed for statistical significance was not achieved. At final analysis (median follow-up of 44♵ months ), median overall survival was not reached (NR 95% CI 49♲-NR) with pembrolizumab vs 36♷ months (27♶-NR) with chemotherapy (hazard ratio 0♷4 95% CI 0♵3-1♰3 p=0♰36). 93 (60%) patients crossed over from chemotherapy to anti-PD-1 or anti-PD-L1 therapy (56 patients to on-study pembrolizumab and 37 patients to off-study therapy). KEYNOTE-177 is registered at, NCT02563002, and is no longer enrolling patients.īetween Feb 11, 2016, and Feb 19, 2018, 852 patients were screened, of whom 307 (36%) were randomly assigned to pembrolizumab (n=153) or chemotherapy (n=154). The co-primary endpoints were overall survival and progression-free survival in the intention-to-treat population. Patients receiving chemotherapy could cross over to pembrolizumab for up to 35 treatment cycles after progression. Patients were randomly assigned (1:1) in blocks of four using an interactive voice response system or integrated web response system to intravenous pembrolizumab 200 mg every 3 weeks or to the investigator's choice of intravenous mFOLFOX6 (oxaliplatin 85 mg/m 2 on day 1, leucovorin 400 mg/m 2 on day 1, and fluorouracil 400 mg/m 2 bolus on day 1 followed by a continuous infusion of 1200 mg/m 2 per day for 2 days on days 1-2) or intravenous FOLFIRI (irinotecan 180 mg/m 2 on day 1, leucovorin 400 mg/m 2 on day 1, and fluorouracil 400 mg/m 2 bolus on day 1 followed by a continuous infusion of 1200 mg/m 2 per day for 2 days on days 1-2), every 2 weeks with or without intravenous bevacizumab 5 mg/kg every 2 weeks or intravenous weekly cetuximab (first dose 400 mg/m 2, then 250 mg/m 2 for every subsequent dose). We recruited patients aged at least 18 years, with an Eastern Cooperative Oncology Group performance status of 0 or 1, and who had previously untreated microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer. This randomised, open-label, phase 3 study was done in 193 academic medical centres and hospitals in 23 countries.

Here, we present the final overall survival analysis of the KEYNOTE-177 study. However, the treatment's effect on overall survival in this cohort of patients was unknown. Pembrolizumab has shown improved progression-free survival versus chemotherapy in patients with newly diagnosed microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer. 18 Sorbonne Université, Hospital Saint Antoine and INSERM 938 and SIRIC CURAMUS, Paris, France.15 National Cancer Center Hospital East, Chiba, Japan.14 Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, USA.13 Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Institute of Oncology, Barcelona, Spain.

12 Hospital Universitario Marques de Valdecilla, IDIVAL, Santander, Spain.10 Western Health, St Albans, VIC, Australia.9 Hospital Universitario Regional y Virgen de la Victoria, IBIMA, Málaga, Spain.8 Oncology Department, Hospital Universitario 12 de Octubre, Imas 12, UCM, Madrid, Spain.7 Bordeaux University Hospital, Bordeaux, France.6 Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands.5 University Hospital of Southern Denmark, Vejle, Denmark.4 Herlev and Gentofte Hospital, Herlev, Denmark.3 Asan Medical Center, University of Ulsan, Seoul, South Korea.Electronic address: 2 University College Hospital, NHS Foundation Trust, London, UK. 1 Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.


 0 kommentar(er)
0 kommentar(er)
